Diseased Liver Model Facilitates Search for AAT Deficiency Cure
By LabMedica International staff writers
Posted on 02 Nov 2017
Researchers have developed a new humanized liver mouse model with the mutation that causes alpha-1 antitrypsin deficiency in order to determine whether it is possible to cure the disease by repopulating the defective liver with gene edited healthy cells.Posted on 02 Nov 2017
In alpha-1 antitrypsin (AAT) deficiency, one missense mutation results in impaired secretion of AAT. In most patients, lung damage occurs due to a lack of AAT-mediated protection of lung elastin from neutrophil elastase. In some patients, accumulation of misfolded PiZ-mutant AAT protein in the liver triggers hepatocyte injury leading to inflammation and cirrhosis.
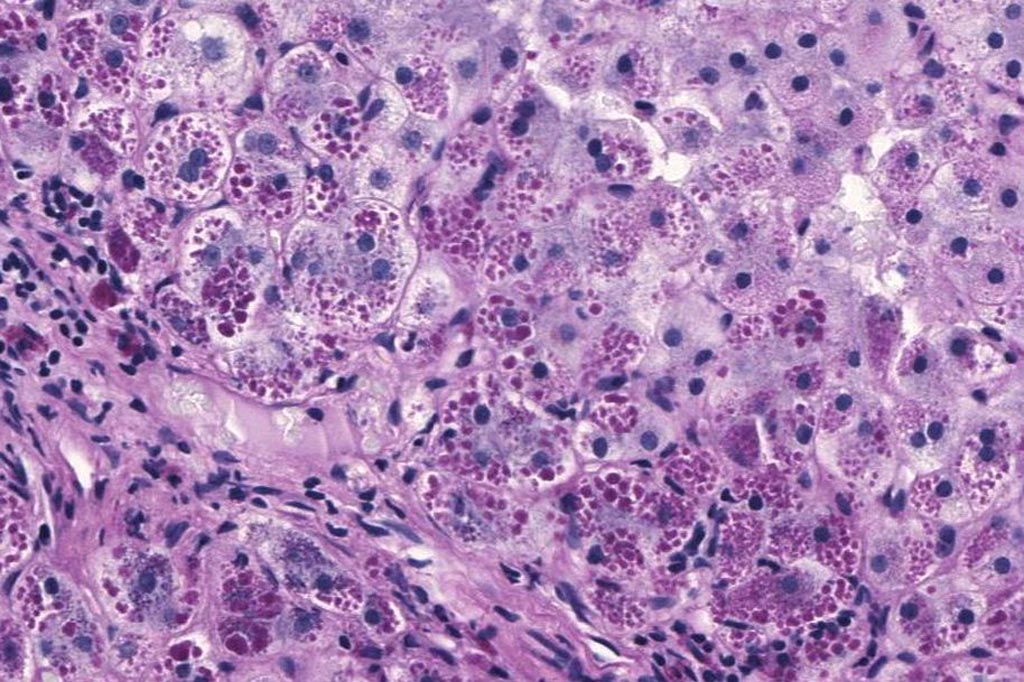
Image: A photomicrograph of a liver biopsy from a patient with alpha-1 antitrypsin deficiency. The PAS with diastase stain shows the diastase-resistant pink globules that are characteristic of this disease (Photo courtesy of Wikimedia Commons).
Investigators at the University of Massachusetts Medical School (Worcester, USA) hypothesized that correcting the mutant defect in hepatocytes would confer a selective advantage for repopulation of hepatocytes within an intact liver. To test this hypothesis they used a combination of RNA interference with gene augmentation, using an RNAi-resistant version of the alpha-1 antitrypsin gene.
The investigators reported in the September 25, 2017, online edition of the journal Molecular Therapy that this gene editing approach led to a selective advantage of edited hepatocytes due to silencing of the mutant protein, augmenting normal AAT production, and improvement of the liver pathology.
"This is a significant win for gene editing," said senior author Dr. Christian Mueller, associate professor of pediatrics at the University of Massachusetts Medical School. "If healthy or gene-corrected liver cells have a selective advantage over cells with the alpha-1 antitrypsin deficiency mutation, then it is possible that by treating only a few cells, those healthy cells will "out compete" the diseased cells. And because liver cells regenerate easily, this can create a big advantage therapeutically. What we have here is a proof of concept that this approach would potentially help patients, and for very young patients with actively growing livers that could potentially be treated early in life, this could be very meaningful"
"Gene editing with alpha-1 antitrypsin deficiency alone can do a lot of what CRISPR/Cas9 [currently the most widely-studied gene editing tool] does, just at a lower efficiency," said Dr. Mueller. "In cases where there is a competitive advantage, only a low-level of editing is necessary, allowing the corrected cells to expand and, in this case, both prevent liver disease and make therapeutic levels of the normal alpha-1 protein."
Related Links:
University of Massachusetts Medical School













