Urine Test to Revolutionize Lyme Disease Testing
Posted on 03 May 2024
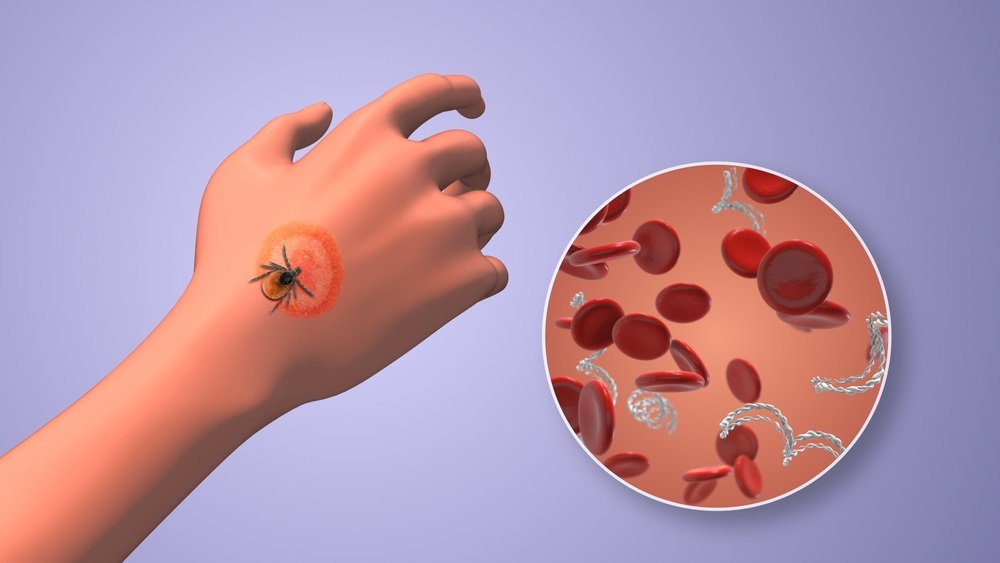
Lyme disease is the most common animal-to-human transmitted disease in the United States, with around 476,000 people diagnosed and treated annually, and its incidence has been increasing. If not addressed promptly and effectively, those affected may experience persistent symptoms known as Post-Treatment Lyme Disease Syndrome, which include issues with concentration and memory, dizziness, fatigue, body aches, depression, and sleep disturbances. Currently, the standard diagnostic approach involves an antibody blood test that detects the immune system's response to the bacteria responsible for Lyme disease. Now, urine testing could revolutionize Lyme disease detection and diagnosis thanks to its many advantages over existing techniques.
Researchers at George Mason University (Fairfax, VA, USA) have developed a urine test for Lyme disease that detects the Borrelia bacteria, which causes Lyme disease. This advancement allows for the confirmation of an infection shortly after a tick bite, leading to timely treatment and helping to avoid the disease's long-term debilitating effects. The test specifically targets molecules originating from the bacteria, ensuring high accuracy and the ability to detect the infection early. The test matches the exact amino acid sequences unique to Borrelia, enhancing its specificity. During clinical trials, this urine test demonstrated a 90% true positive rate and nearly 100% specificity.
The team is currently conducting a three-year study using previously collected samples from both cross-sectional and longitudinal studies involving patients with acute Lyme disease. This study will also trial a new, collapsible urine collection cup that can be mailed to a laboratory, simplifying the collection process and making diagnostic services more accessible via telehealth. This innovative collection method allows patients to comfortably and privately collect samples at home, ensuring the accuracy of lab tests. The urine cups are shipped in a semi-dry state that preserves target proteins and prevents specimen degradation, addressing the specificity challenges of previous testing methods.
“This is a significant collaboration to advance diagnostics for Lyme disease. In my capacity as an epidemiologist, I am thrilled to work with Drs. Luchini, Liotta, and Espina, and Dr. Krall in her capacity as a biostatistician. This study will have a major impact on the timely diagnosis of Lyme,” said Melissa J. Perry, dean of the College of Public Health and co-investigator of the study.
Related Links:
George Mason University










_1.jpg)
 (1)_1.jpg)