Genome Editing Used to Bias Genetic Inheritance in Mice
By LabMedica International staff writers
Posted on 06 Feb 2019
Genomics researchers have demonstrated the feasibility of using CRISPR/Cas9 gene editing to bias genetic inheritance in mice used for laboratory studies.Posted on 06 Feb 2019
Investigators at the University of California, San Diego (USA) based their work in mice on the highly efficient gene drive systems that have recently been developed in insects. These systems leveraged the sequence-targeted DNA cleavage activity of CRISPR/Cas9 and endogenous homology-directed DNA repair mechanisms to convert heterozygous genotypes to homozygosity. The investigators postulated that if implemented in laboratory rodents, similar systems would enable the rapid assembly of currently impractical genotypes that involve multiple homozygous genes (for example, to model multigenic human diseases).
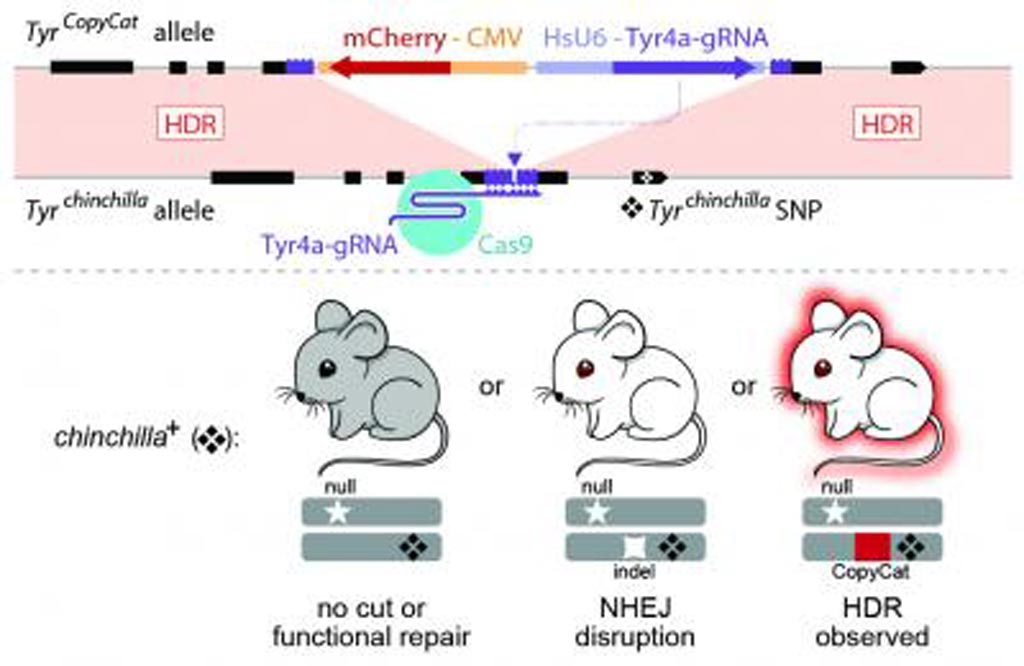
Image: Researchers used CRISPR/Cas9 genome editing to control genetic inheritance in mammals for the first time (Photo courtesy of Cooper Laboratory, University of California, San Diego).
Towards this end, the investigators used an active genetic element that encoded a guide RNA, which was embedded in the mouse tyrosinase (Tyr) gene, to evaluate whether targeted gene conversion could occur when CRISPR/Cas9 was active in the early embryo or in the developing germline.
CRISPR/Cas9 is regarded as the cutting edge of molecular biology technology. CRISPRs (clustered regularly interspaced short palindromic repeats) are segments of prokaryotic DNA containing short repetitions of base sequences. Each repetition is followed by short segments of "spacer DNA" from previous exposures to a bacterial virus or plasmid. Since 2013, the CRISPR/Cas9 system has been used in research for gene editing (adding, disrupting, or changing the sequence of specific genes) and gene regulation. By delivering the Cas9 enzyme and appropriate guide RNAs (sgRNAs) into a cell, the organism's genome can be cut at any desired location. The conventional CRISPR/Cas9 system from Streptococcus pyogenes is composed of two parts: the Cas9 enzyme, which cleaves the DNA molecule and specific RNA guides that shepherd the Cas9 protein to the target gene on a DNA strand.
The investigators reported in the January 23, 2019, online edition of the journal Nature that while Cas9 efficiently induced double-stranded DNA breaks in the early embryo and male germline, these breaks were not corrected by homology-directed repair and the modified gene was not passed on. In contrast, Cas9 expression limited to the female germline induced double-stranded breaks that were corrected by homology-directed repair, which copied the active genetic element from the donor to the receiver chromosome and increased its rate of inheritance in the next generation. In one study, the target gene was inherited from the female parent in as many as 86% of offspring instead of the usual 50%. It was not obvious why modifications of the female germline were inherited while modifications of the male germline were not.
"Our motivation was to develop this as a tool for laboratory researchers to control the inheritance of multiple genes in mice," said senior author Dr. Kimberly L. Cooper, assistant professor of biology at the University of California, San Diego. "With further development we think it will be possible to make animal models of complex human genetic diseases, like arthritis and cancer that are not currently possible."
"We have shown that we can convert one genotype from heterozygous to homozygous. Now we want to see if we can efficiently control the inheritance of three genes in an animal. If this can be implemented for multiple genes at once, it could revolutionize mouse genetics," said Dr. Cooper. "We are also interested in understanding the mechanisms of evolution. For certain traits that have evolved over tens of millions of years, the number of genetic changes is greater than we can currently assemble in mice to understand what caused bat fingers to grow into a wing, for example. So we want to make lots of these active genetic tools to understand the origins of mammalian diversity."
Related Links:
University of California, San Diego













