Preventing Accumulation of Senescent Cells Reverses Adverse Signs of Aging
By LabMedica International staff writers
Posted on 15 Jan 2019
Researchers working with mouse models have shown that some of the less desirable signs of aging, such as chronic inflammation and reduced function of some organs, could be reversed by treatment to reduce the number of senescent cells that have accumulated in the animal.Posted on 15 Jan 2019
Senescent cells are aged or damaged cells that accumulate in tissues in advanced age. They no longer are able to perform their normal roles and interfere with the functioning of the tissue in which they accumulate. Elimination of senescent cells is considered to be a promising therapeutic approach.
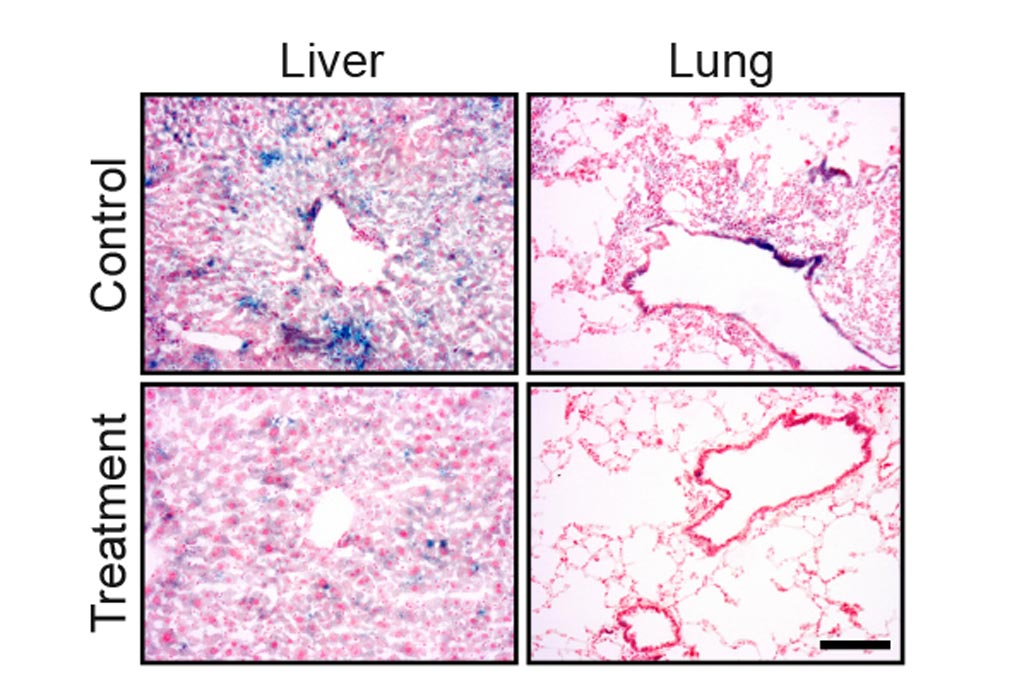
Image: Drug treatment eliminates senescent cells from tissues of old mice. The blue staining shows senescent cells in lung and liver tissue. The amount of the staining is significantly reduced following the drug treatment (Photo courtesy of The Weizmann Institute of Science).
The extent of immune-system involvement in regulating age-related accumulation of senescent cells, and its consequences, are unknown. To evaluate the role of the immune system in the aging process, investigators at the Weizmann Institute of Science (Rehovot, Israel) worked with Prf1−/− mice with impaired cell cytotoxicity, which suffered from chronic inflammation, and with progeroid (progeroid means "resembling premature aging") mice with impaired cell cytotoxicity that promoted senescent-cell accumulation and shortened lifespan.
The investigators reported in the December 21, 2018, online edition of the journal Nature Communications that Prf1−/− mice with impaired cell cytotoxicity exhibited both higher senescent cell tissue burden and chronic inflammation. They suffered from multiple age-related disorders and lower survival. The accumulation of senescent cells in these Prf1−/− mice was accompanied by a progressive state of chronic inflammation, followed by increased tissue fibrosis and other types of tissue damage, as well as compromised organ functionality. The poor health of old Prf1−/− mice was associated with fitness reduction, weight loss, kyphosis (abnormally excessive convex curvature of the spine), older appearance, and shorter lifespan than that of wild type controls.
The investigators reported that elimination of senescent cells from old Prf1−/− mice could be achieved by pharmacological inhibitors of the BCL-2 family of proteins, such as ABT-737. First developed for potential cancer chemotherapy, ABT-737 was subsequently identified as a senolytic (a drug that selectively induces cell death in senescent cells). This pharmacological approach attenuated age-related phenotypes and gene expression profile in Prf1−/− mice. Furthermore, implementation of this approach on Prf1−/− progeroid mice increased median lifespan of these animals.
These findings shed new light on mechanisms governing senescent-cell presence in aging, and could motivate new strategies for regenerative medicine.
Related Links:
Weizmann Institute of Science













