Protein Crystals Used to Prepare Cellular Drug Delivery Nanotubes
By LabMedica International staff writers
Posted on 28 Nov 2018
A team of Japanese biomolecular engineers used scaffolds of cross-linked protein crystals to prepare nanotubes, which are structures that can serve as the basis for minute delivery systems for drugs and other substances.Posted on 28 Nov 2018
Investigators at the Tokyo Institute of Technology (Japan) reported in the October 30, 2018, online edition of the journal Chemical Science that they had developed a new method for preparing nano-size structures using protein crystals as non-equilibrium molecular scaffolds. Protein crystals were found to provide an ideal environment with a highly ordered packing of subunits in which the supramolecular assembled structures formed in the crystalline matrix.
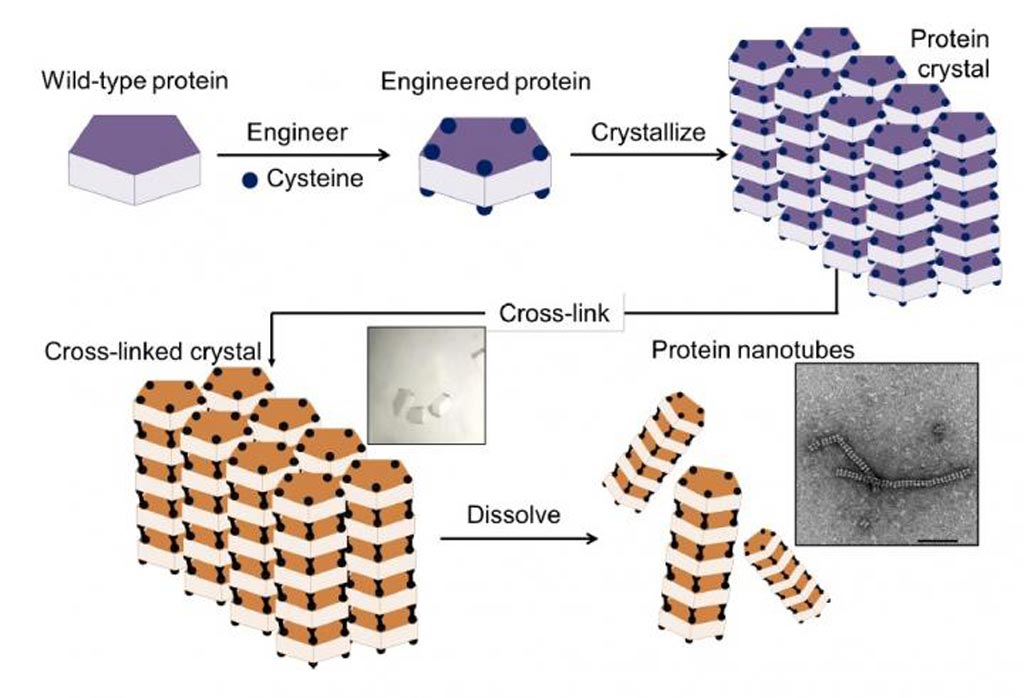
Image: The method for assembly of protein nanotubes involved a four-step process: 1) introduction of cysteine residues into the wild-type protein; 2) crystallization of the engineered protein into a lattice structure; 3) formation of a cross-linked crystal; and 4) dissolution of the scaffold to release the protein nanotubes (Photo courtesy of Chemical Science).
The investigators used the protein RubisCO (Ribulose-1,5-bisphosphate carboxylase/oxygenase) as the building block for construction of the nanotubes. This protein was selected for its high stability, which enabled it to retain its shape and crystal structure.
As described, the method comprised four steps: (1) Preparation of an engineered protein; (2) formation of the protein crystal scaffold; (3) formation of a cross-linked crystal; (4) release of the protein nanotubes by dissolving the scaffold. The assembly of tubes was driven by the formation of disulfide bonds to retain the intermolecular interactions within each assembly in the crystalline matrix after dissolution of the crystals. Transmission electron microscopy (TEM) was used to confirm the formation of the protein nanotubes.
"Our cross-linking method can facilitate the formation of the crystal scaffold efficiently at the desired position (specific cysteine sites) within each tube of the crystal," said senior author Dr. Takafumi Ueno, professor of biomolecular engineering at the Tokyo Institute of Technology. "At present, since more than 100,000 protein crystal structures have been deposited in protein data bank, our method can be applied to other protein crystals for construction of supramolecular protein assemblies, such as cages, tubes, sheets."
Related Links:
Tokyo Institute of Technology