Gene Editing Reverses DMD Muscle Damage in Model
By LabMedica International staff writers
Posted on 10 Sep 2018
The CRISPR/Cas9 genome-editing tool was used in a proof-of-concept study conducted in dogs suggested that the technique could be clinically useful for the treatment of Duchenne muscular dystrophy.Posted on 10 Sep 2018
Duchenne muscular dystrophy (DMD) is caused by mutations in the gene that encodes dystrophin, a protein crucial for maintaining muscle cell integrity and function, and the subsequent disruption of the dystrophin-associated protein complex (DAPC). There are more than 3000 different mutations in the X-chromosome-linked dystrophin gene, and the disease effects about one of every 3,500 boys whose functioning of cardiac and skeletal muscle is so degraded that they die usually before reaching the age of 30. The majority of DMD mutations are deletions that prematurely terminate the dystrophin protein.
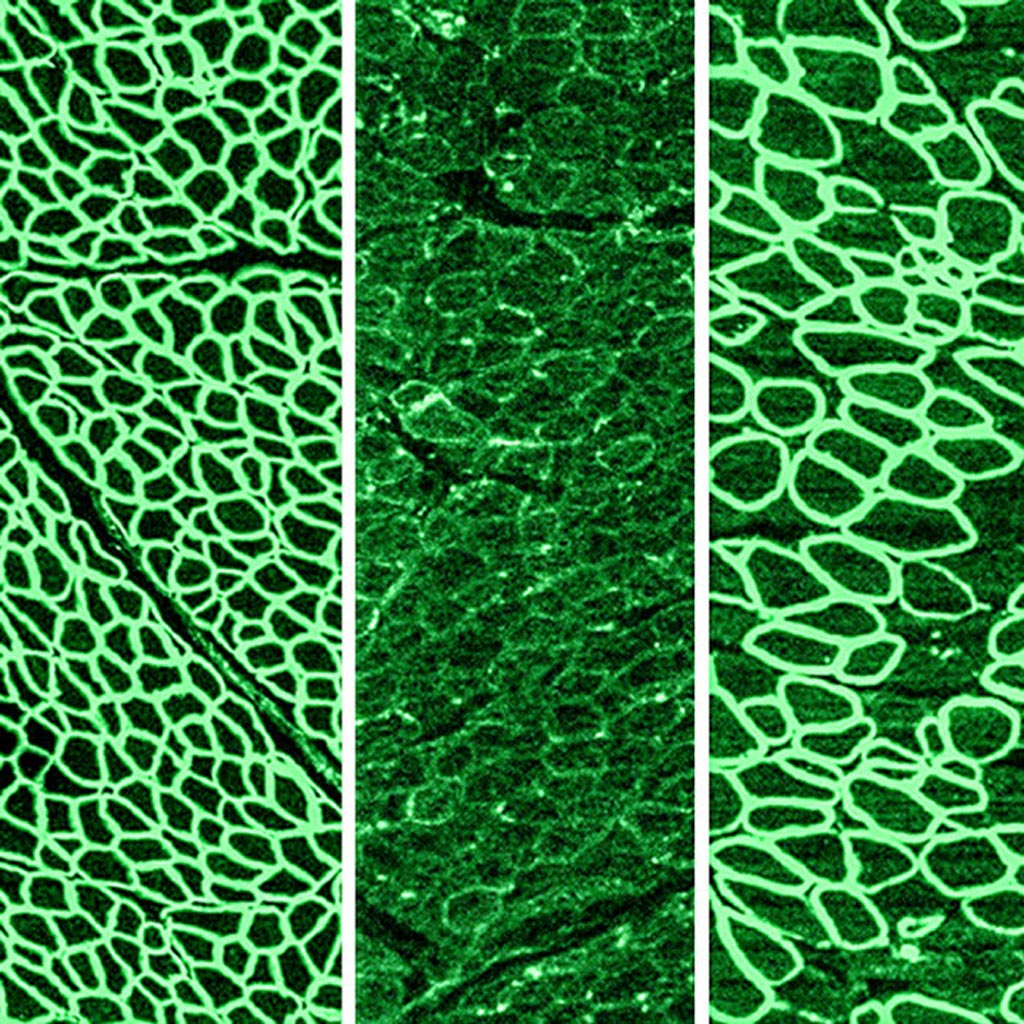
Image: In a recent study, CRISPR gene editing was shown to halt the progression of Duchenne muscular dystrophy (DMD) in dogs. The photomicrographs show dystrophin (in green) in a healthy diaphragm muscle (left), absence of dystrophin in a dog with DMD (center), and restoration of dystrophin in dogs treated with CRISPR (right) (Photo courtesy of the University of Texas Southwestern Medical Center).
CRISPR/Cas9 is regarded as the cutting edge of molecular biology technology. CRISPRs (clustered regularly interspaced short palindromic repeats) are segments of prokaryotic DNA containing short repetitions of base sequences. Each repetition is followed by short segments of "spacer DNA" from previous exposures to a bacterial virus or plasmid. Since 2013, the CRISPR/Cas9 system has been used in research for gene editing (adding, disrupting, or changing the sequence of specific genes) and gene regulation. By delivering the Cas9 enzyme and appropriate guide RNAs (sgRNAs) into a cell, the organism's genome can be cut at any desired location. The conventional CRISPR/Cas9 system is composed of two parts: the Cas9 enzyme, which cleaves the DNA molecule and specific RNA guides that shepherd the Cas9 protein to the target gene on a DNA strand. Efficient genome editing with Cas9-sgRNA in vivo has required the use of viral delivery systems, which have limitations for clinical applications.
In a previous study, investigators at the University of Texas Southwestern Medical Center (Dallas, USA) demonstrated that CRISPR/Cas9 editing could reverse the DMD-causing mutation in mice. In the current study, they worked with the deltaE50-MD dog model of DMD, which harbors a mutation corresponding to a mutational “hot spot” in the human DMD gene.
The investigators used adeno-associated viruses to deliver CRISPR gene editing components to four dogs and examined dystrophin protein expression six weeks after intramuscular delivery (two of the dogs) or eight weeks after systemic delivery (the other two dogs).
Results published in the August 30, 2018, online edition of the journal Science revealed that after systemic delivery in skeletal muscle, dystrophin was restored to levels ranging from 3% to 90% of normal, depending on muscle type. In cardiac muscle, dystrophin levels in the dog receiving the highest dose reached 92% of normal. The treated dogs also showed improved muscle histology.
"Children with DMD often die either because their heart loses the strength to pump, or their diaphragm becomes too weak to breathe," said senior author Dr. Eric Olson, professor of molecular biology at the University of Texas Southwestern Medical Center. "This encouraging level of dystrophin expression would hopefully prevent that from happening."
Related Links:
University of Texas Southwestern Medical Center













