PARP-Inhibitors Reduce Damage in Some Neurological Disorders
By LabMedica International staff writers
Posted on 21 Aug 2018
A recent study found that blocking the activity of the poly(ADP-ribose)polymerase (PARP) family of enzymes reduced the amount of harmful TDP-43 (transactive response DNA binding protein) structures in brain cells under stress and slowed or prevented development of cytoplasmic inclusions found in neurological disorders such as amyotrophic lateral sclerosis (ALS), and some forms of frontotemporal degeneration (FTD).Posted on 21 Aug 2018
In ALS and FTD, cytoplasmic aggregates of hyperphosphorylated TDP-43 accumulate and co-localize with some stress granule components, but how pathological TDP-43 aggregation is transferred to the nucleus remains unknown.
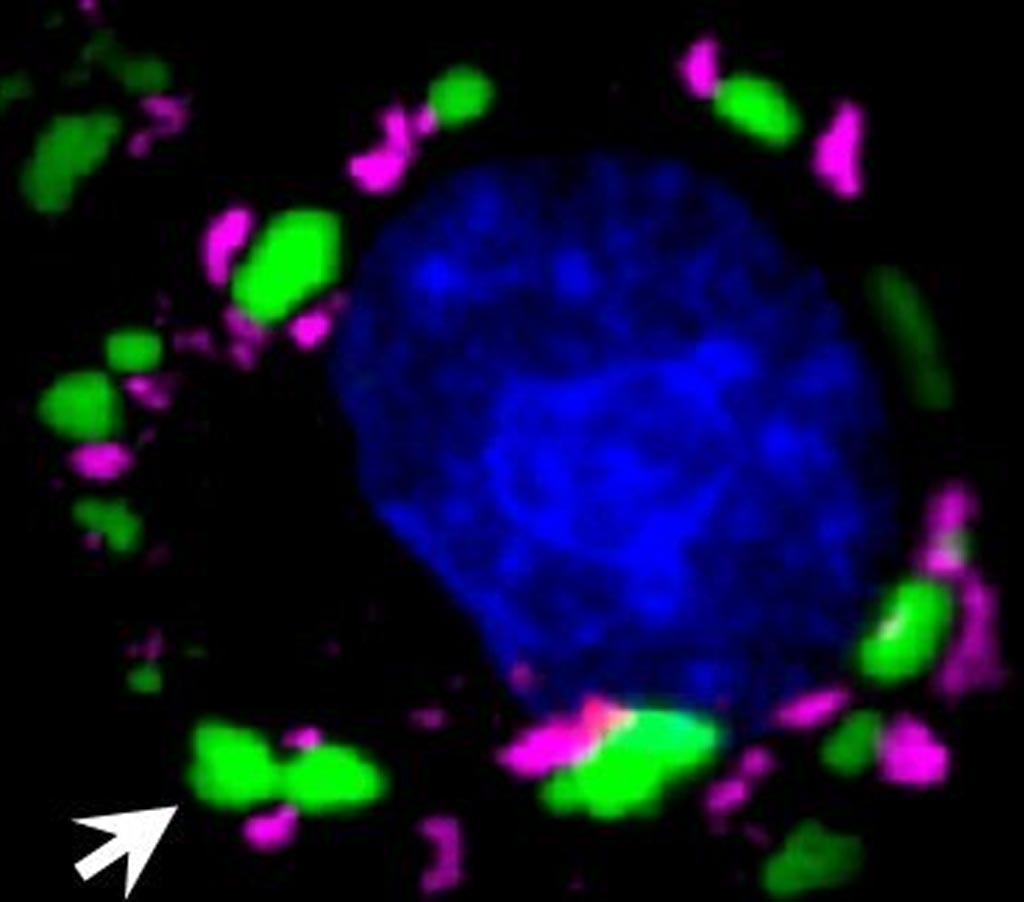
Image: In cells under duress, stress granules (in magenta) form outside of the nucleus (in blue). TDP-43 protein in green (arrow) that cannot bind to polyADP ribose (PAR) builds up in large clumps distinct from stress granules (Photo courtesy of Leeanne McGurk, University of Pennsylvania).
Investigators at the University of Pennsylvania (Philadelphia, USA) used cell cultures and a Drosophila (fruit fly) model to show that downregulation of the enzyme tankyrase, a PAR polymerase, reducedTDP-43 accumulation in the cytoplasm and potently inhibited neurodegeneration. They established that TDP-43 non-covalently bound to PAR via PAR-binding motifs embedded within its nuclear localization sequence. PAR binding promoted liquid-liquid phase separation of TDP-43 in vitro and was required for TDP-43 accumulation in stress granules in mammalian cells and neurons. Stress granule localization initially protected TDP-43 from disease-associated phosphorylation, but upon long-term stress, stress granules eventually dissolved, leaving behind aggregates of phosphorylated TDP-43.
The investigators reported in the August 9, 2018, online edition of the journal Molecular Cell that PARP inhibitors, which stop PAR from being generated, reduced the amount of harmful TDP-43 structures localized into stress granules. This reduced TDP-43-linked pathology and neurodegeneration and could be of use therapeutically for the treatment of ALS and FTD.
“Given the lack of treatment options, we are excited by these experiments that help elucidate molecular events that could lead to new therapeutics,” said senior author Dr. Nancy Bonini, professor of biology at the University of Pennsylvania.
Related Links:
University of Pennsylvania













