Genetic Code Expansion Used to Establish Biological Control Mechanism
By LabMedica International staff writers
Posted on 17 Jul 2018
A team of genomics researchers has described the development of a novel biological switching mechanism that enables control over the activity of the CRISPR/Cas9 gene-editing tool.Posted on 17 Jul 2018
CRISPR/Cas9 is regarded as the cutting edge of molecular biology technology. CRISPRs (clustered regularly interspaced short palindromic repeats) are segments of prokaryotic DNA containing short repetitions of base sequences. Each repetition is followed by short segments of "spacer DNA" from previous exposures to a bacterial virus or plasmid. Since 2013, the CRISPR/Cas9 system has been used in research for gene editing (adding, disrupting, or changing the sequence of specific genes) and gene regulation. By delivering the Cas9 enzyme and appropriate guide RNAs (sgRNAs) into a cell, the organism's genome can be cut at any desired location. The conventional CRISPR/Cas9 system is composed of two parts: the Cas9 enzyme, which cleaves the DNA molecule and specific RNA guides that shepherd the Cas9 protein to the target gene on a DNA strand.
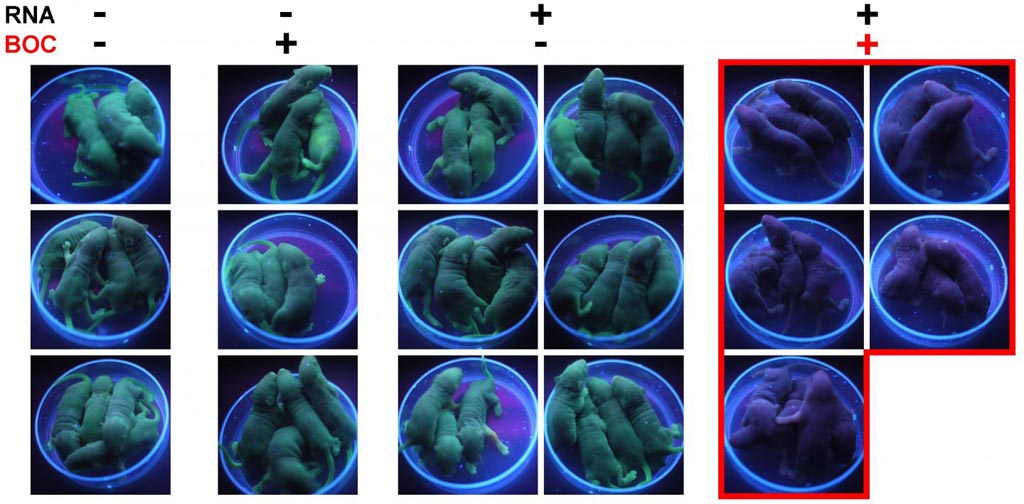
Image: The left-side group of mice is a control - with neither the gene editing RNA, nor the BOC switch. The next group has BOC but no RNA, and the third has the RNA, but no BOC to switch it on. The final group on the right has the gene editing machinery and the BOC switch. BOC activates the switch and the pups are born without green fluorescence (Photo courtesy of Dr. Tony Perry, University of Bath).
Multiple applications of genome editing by CRISPR-Cas9 necessitate stringent regulation and Cas9 variants have accordingly been generated whose activity responds to small ligands, temperature, or light. However, these approaches are often impracticable, for example in clinical therapeutic genome editing in situ or gene drives in which environmentally compatible control is paramount. Clearly, Cas9 regulation by other means will be advantageous for many of its potential applications.
To address this issue, investigators at the University of Bath (United Kingdom) and Cardiff University (United Kingdom) speculated that a cheap amino acid such as the lysine derivative, H-Lys(Boc)-OH (BOC), might be harnessed for Cas9 control. BOC can be incorporated into proteins of interest by genetic code expansion.
An expanded genetic code is an artificially modified genetic code in which one or more specific codons have been re-allocated to encode an amino acid that is not among the 20 common naturally encoded proteinogenic amino acids. The key prerequisites to expand the genetic code are: the non-standard amino acid to encode, an unused codon to adopt, a tRNA (transfer RNA) that recognizes this codon, and a tRNA synthetase enzyme that recognizes only that tRNA and only the non-standard amino acid. Expanding the genetic code is an area of research of synthetic biology, an applied biological discipline whose goal is to engineer living systems for useful purposes.
The investigators reported in the July 3, 2018, online edition of the journal Scientific Reports that they had developed a method of heritable Cas9-mediated mammalian genome editing that was acutely controlled by the cheap lysine derivative, Lys(Boc). Genetic code expansion permitted non-physiological BOC incorporation such that Cas9 (Cas9BOC) was expressed in a full-length, active form in cultured somatic cells only after BOC exposure.
To demonstrate the principle of Cas9BOC gene editing, the investigators used a transgenic mouse model that carried a gene that caused the animals' skin to glow green under UV light. When the CAS9BOC genome-editing tool was present in embryos from these mice, their genomic DNA was efficiently edited to remove the fluorescence gene, but only when BOC was present. In the absence of BOC, no editing occurred.
Senior author Dr. Anthony Perry, professor of biology and biochemistry at the University of Bath, said, "Our switch is a way of controlling the expression of any protein via genetic code expansion. What sets our work apart is the potential for this as an environmentally friendly switch across large distances, which no previous method really enables. For example you can imagine controlling gene drive activity in livestock herds by adding or removing BOC from feedstuffs as required. Gene editing has enormous potential across biological science, from biomedicine to food security, in insects, plants and animals."
Related Links:
University of Bath
Cardiff University













