Novel Use of Nanoparticles Developed for Targeted Drug Delivery
By LabMedica International staff writers
Posted on 26 Jun 2018
The innovative use of metal-organic framework nanoparticles has enabled the development of a delivery system for therapeutic proteins that selectively targets tumor cells.Posted on 26 Jun 2018
Metal-organic frameworks (MOFs) are a class of crystalline materials that consist of coordination bonds between transition-metal cations and multidentate organic linkers. MOFs are made by linking inorganic and organic units by strong bonds (reticular synthesis), and the structure of MOFs is characterized by an open framework that can be porous. MOFs are known for their extraordinarily high surface areas, tunable pore size, and adjustable internal surface properties. The flexibility with which the constituents’ geometry, size, and functionality can be varied has led to more than 20,000 different MOFs being reported and studied within the past decade.
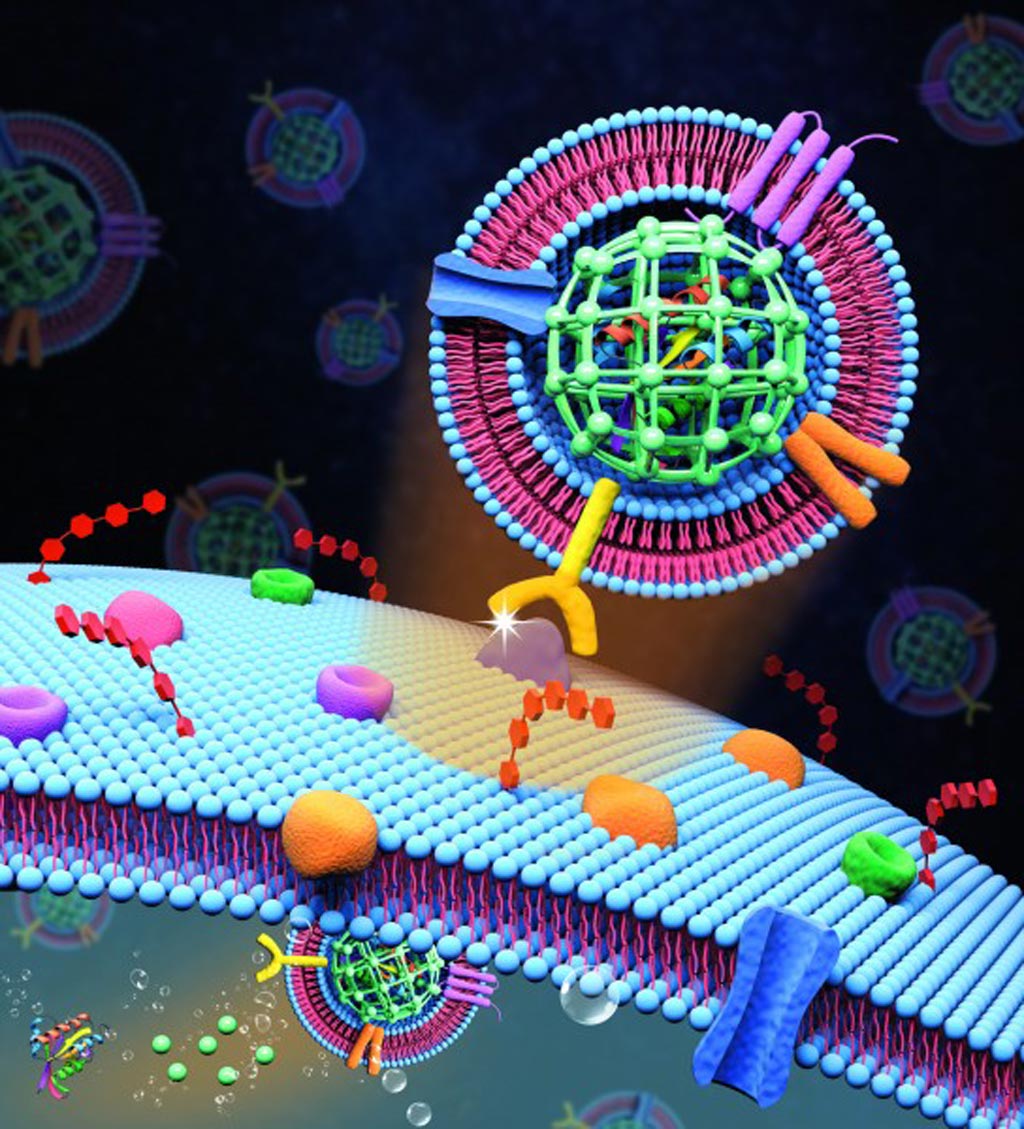
Image: Extracellular vesicle-like metal-organic framework nanoparticles are developed for the intracellular delivery of biofunctional proteins. The biomimetic nanoplatform can protect the protein cargo and overcome various biological barriers to achieve systemic delivery and autonomous release (Photo courtesy of the Zheng Laboratory, Pennsylvania State University).
Current strategies for transporting protein therapeutic agents face substantial challenges owing to various biological barriers, including susceptibility to protein degradation and denaturation, poor cellular uptake, and low transduction efficiency into the cytosol.
To counter these difficulties, investigators at Pennsylvania State University (State College, USA) developed a biomimetic nanoparticle platform for systemic and intracellular delivery of proteins. Using a biocompatible strategy, they caged guest proteins in the matrix of metal–organic frameworks with high efficiency (up to about 94%) and high loading content (the protein toxin gelonin at up to about 50 times those achieved by surface conjunction), and the nanoparticles were further decorated with tumor cell extracellular vesicle (EV) membranes with an efficiency as high as nearly 97%.
The nanoparticles, which were selectively transported to the tumor site due to the property of homotypic targeting engendered by the EV membrane coating, were taken up by the cancer cells through endocytosis. Once inside the cells, the higher acidity of the cancer cell’s intracellular transport vesicles degraded the MOF nanoparticles, which released the toxic protein into the cytosol and killed the cells.
Results of in vitro and in vivo studies published in the May 29, 2018, online edition of the Journal of the American Chemical Society revealed that the EV-like nanoparticles not only protected proteins against protease digestion and evaded immune system clearance but also selectively targeted homotypic tumor sites and promoted tumor cell uptake and autonomous release of the guest protein after internalization. Using this novel nanoparticle transport mechanism to deliver the bioactive therapeutic protein gelonin in a mouse model system significantly inhibited tumor growth and increased therapeutic efficacy14-fold.
“We designed a strategy to take advantage of the extracellular vesicles derived from tumor cells," said senior author Dr. Siyang Zheng, associate professor of biomedical and electrical engineering at Pennsylvania State University. "We remove 99% of the contents of these extracellular vesicles and then use the membrane to wrap our metal-organic framework nanoparticles. If we can get our extracellular vesicles from the patient, through biopsy or surgery, then the nanoparticles will seek out the tumor through a process called homotypic targeting. Our metal-organic framework has very high loading capacity, so we do not need to use a lot of the particles and that keeps the general toxicity low."
Related Links:
Pennsylvania State University













