Oral Cambinol Treatment Shows Potential for Blocking Development of AD
By LabMedica International staff writers
Posted on 16 May 2018
Alzheimer's disease researchers have shown that the low molecular weight drug cambinol blocks the formation of exosomes that allow the spread of insoluble, toxic tau aggregates, which are characteristic of the neurodegenerative disorder.Posted on 16 May 2018
Tau proteins are components of the cytoskeleton of neurons, but in Alzheimer's disease, tau proteins become abnormally modified and condense into insoluble "neurofibrillary tangles" that destroy these brain cells. Furthermore, dying cells encase tau aggregates in exosome lipid vesicles, which bud off and "seed" neighboring tissues, spreading the disease.
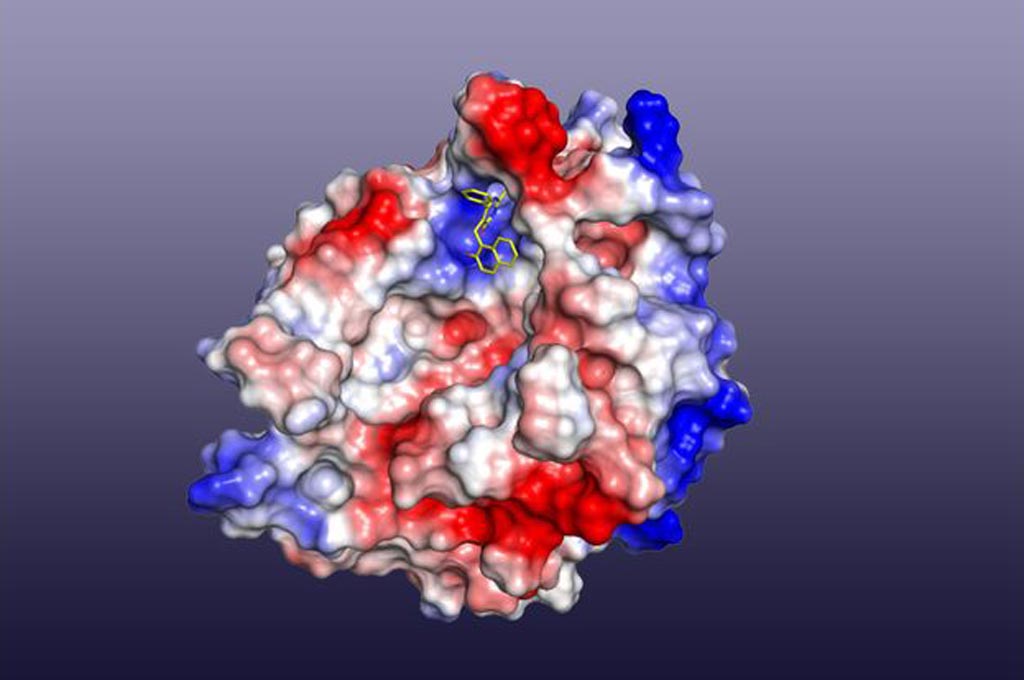
Image: A model of cambinol (small yellow structure near top center) binding to nSMAse2 enzyme (Photo courtesy of Kanagasabai Vadive, University of California, Los Angeles).
During a screen of potential drug candidates capable of blocking the seeding of tau tangles, investigators at the University of California, Los Angeles (USA), identified the compound cambinol. Previous studies had shown that cambinol was a novel uncompetitive inhibitor of the enzyme nSMase2 (neutral sphingomyelinase 2). The enzyme nSMase2 had been found to be critically required for catalyzing the production of exosome vesicles.
The inhibitory activity of cambinol for nSMase2 was approximately 10-fold more potent than for its previously known target, SIRT1/2 (silence information regulator 1 and 2). Cambinol decreased tumor necrosis factor-alpha or interleukin-1 beta-induced increases of ceramide and cell death in primary neurons. A preliminary study of cambinol structure and activity identified the main structural features required for nSMase2 inhibition.
In a paper published in the April 9, 2018, online edition of the journal Biochemical and Biophysical Research Communications, the investigators demonstrated that cambinol was an inhibitor of cell-to-cell tau propagation. In vitro data revealed that cambinol inhibited nSMase2 enzyme activity in dose response fashion, and suppressed exosome vesicle production while reducing tau seed propagation. In vivo testing in mice showed that cambinol could reduce the nSMase2 activity in the brain after oral administration.
"Over 200 molecules have been tested as disease-modifying Alzheimer's therapy in clinical trials, and none has yet attained the Holy Grail," said senior author Dr. Varghese John, associate professor of neurology at the University of California, Los Angeles. "Our paper describes a novel approach to slow Alzheimer's progression by showing it is possible to inhibit propagation of pathologic forms of tau."
Decreased nSMase2 catalytic activity was observed in the brains of mice given oral cambinol. "Getting molecules into the brain is a big hurdle, because most drugs do not penetrate the blood-brain barrier, said Dr. John. "Now we know we can treat animals with cambinol to determine its effect on Alzheimer's pathology and progression."
Related Links:
University of California, Los Angeles













