Extracellular Collagen Modulates Tumor Progression and Metastasis
By LabMedica International staff writers
Posted on 05 Dec 2017
A recent paper detailed how factors in the tumor microenvironment induce the cancer to progress and metastasize.Posted on 05 Dec 2017
Previous studies had suggested that the topographical organization of collagen within the tumor microenvironment modulated cancer cell migration and was independently predictive of progression to metastasis.
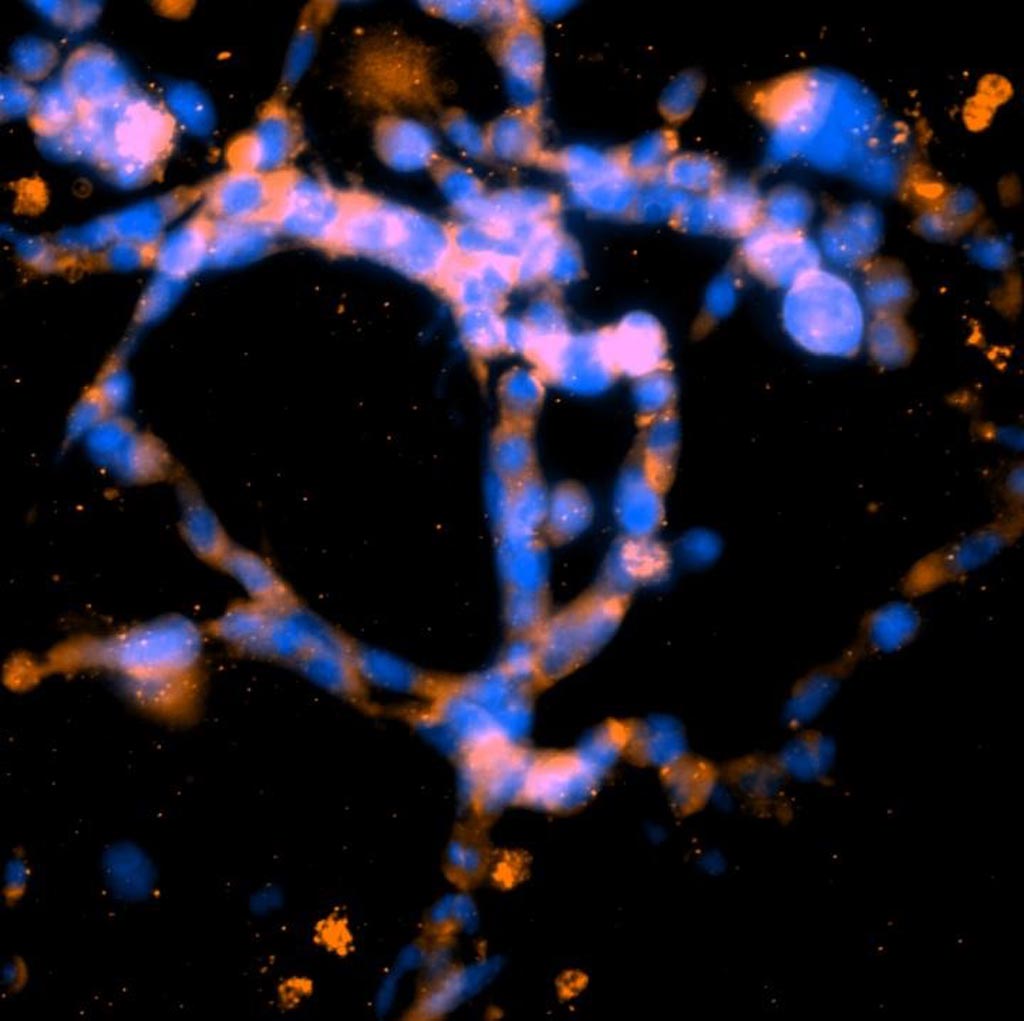
Image: Breast cancer cells were grown in a highly dense three-dimensional collagen matrix. After seven days the cells formed networks that resembled the early stages of blood vessel development. This photomicrograph shows representative structures observed in these environments after staining for cell nuclei (blue) and cytoskeletons (green) (Photo courtesy of the University of California, San Diego).
Investigators at the University of California, San Diego (USA) explored the relationships between collagen density, collagen architecture, cell migration behavior, gene expression, and metastatic potential. To do this, they developed a three-dimensional collagen matrix model system designed to probe the physical basis of cancer cell migration responses to collagen matrix organization.
Results published in the November 21, 2017, online edition of the journal Nature Communications revealed that collagen matrices with small pores and short fibers conserved a transcriptional response and subsequent motility switch in cancer cells resulting in the formation of multicellular network structures. The response was not mediated by hypoxia, matrix stiffness, or bulk matrix density, but rather by matrix architecture-induced beta1-integrin upregulation. The set of genes, or gene module, which caused this behavior, was dubbed the collagen-induced network phenotype (CINP).
The investigators found that this CINP-induced behavior was consistent with phenotypic and molecular features of clinical vasculogenic mimicry (VM) – the formation of structures resembling blood vessels. Furthermore, they showed that the associated transcriptional response was conserved among cancer types in vitro and was predictive of patient survival in multiple clinical datasets for various tumor types. These findings linked a collagen-induced migration program to VM and suggested that this process could be broadly relevant to metastatic progression in solid human cancers.
"It is almost like the matrix is encoding the gene module," explained senior author Dr. Stephanie Fraley, professor of bioengineering at the University of California, San Diego. "The cells do not exhibit this behavior in traditional petri dishes. It is critical to have the cells surrounded by a three-dimensional environment that mimics what happens in the human body."
Related Links:
University of California, San Diego