Novel Peptide Targets Injured Brain Tissues
By LabMedica International staff writers
Posted on 24 Nov 2017
A novel peptide with potential therapeutic use for the treatment of neurological disorders such as Alzheimer's disease (AD) and Parkinson's disease was identified by in vivo phage display screening.Posted on 24 Nov 2017
In vivo peptide phage display can be used for unbiased probing of tissues in situ for specific molecular signatures, particularly in the vasculature. Investigators at Sanford-Burnham Prebys Medical Discovery Institute (La Jolla, CA, USA) utilized this technique to discover homing peptides specific for different pathologies including tumors, atherosclerotic plaques, wounds, and severe brain injury.
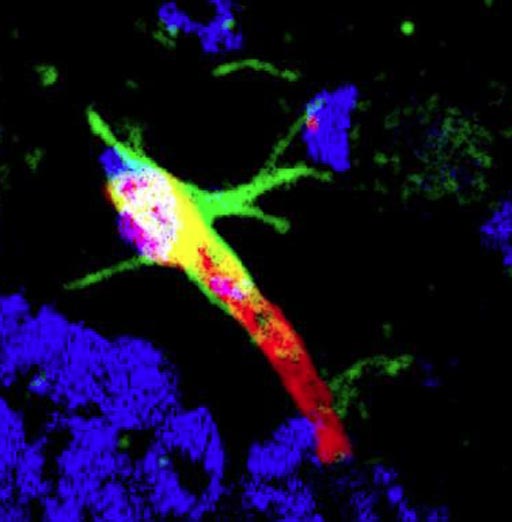
Image: A photomicrograph showing DAG (green-labeled peptide) targeting to the brain blood vessel (labeled red) in the hippocampus of the Alzheimer brain (Photo courtesy of the Ruoslahti Laboratory, Sanford-Burnham Prebys Medical Discovery Institute).
Phage display is a laboratory technique for the study of protein–protein, protein–peptide, and protein-DNA interactions that uses bacteriophages to connect proteins with the genetic information that encodes them. In this technique, a gene encoding a protein of interest is inserted into a phage coat protein gene, causing the phage to "display" the protein on its outside while containing the gene for the protein on its inside, resulting in a connection between genotype and phenotype. These displaying phages can then be screened against other proteins, peptides, or DNA sequences, in order to detect interaction between the displayed protein and those other molecules. In this way, large libraries of proteins can be screened and amplified.
The investigators reported in the November 10, 2017, online edition of the journal Nature Communications that they had identified a cyclic nine amino acid peptide, DAG, which accumulated in the hippocampus of hAPP-J20 mice at different ages.
Intravenously injected DAG peptide homed to neurovascular unit endothelial cells and to reactive astrocytes in mouse models of AD. The investigators identified connective tissue growth factor (CTGF), a matricellular protein (a dynamically expressed non-structural protein that is present in the extracellular matrix) that is highly expressed in the brain of individuals with AD and in mouse models, as the target of the DAG peptide. They also showed that exogenously delivered DAG homed to the brain in mouse models of glioblastoma, traumatic brain injury, and Parkinson’s disease.
"Our findings show that endothelial cells, the cells that form the inner lining of blood vessels, bind our DAG peptide in the parts of the mouse brain affected by the disease," said senior author Dr. Erkki Ruoslahti, a distinguished professor at Sanford-Burnham Prebys Medical Discovery Institute. "This is very significant because the endothelial cells are readily accessible for probes injected into the blood stream, whereas other types of cells in the brain are behind a protective wall called the blood-brain barrier. The change in AD blood vessels gives us an opportunity to create a diagnostic method that can detect AD at the earliest stage possible. But first we need to develop an imaging platform for the technology, using MRI or PET scans to differentiate live AD mice from normal mice. Once that is done successfully, we can focus on humans."
"As our research progresses we also foresee CTGF as a potential therapeutic target that is unrelated to amyloid beta (Abeta), the toxic protein that creates brain plaques," said Dr. Ruoslahti. "Given the number of failed clinical studies that have sought to treat AD patients by targeting Abeta, it is clear that treatments will need to be given earlier--before amyloid plaques appear--or have to target entirely different pathways.
DAG has the potential to fill both roles -- identifying at risk individuals prior to overt signs of AD and targeted delivery of drugs to diseased areas of the brain. Perhaps CTGF itself can be a drug target in AD and other brain disorders linked to inflammation. We will just have to learn more about its role in these diseases."
Related Links:
Sanford-Burnham Prebys Medical Discovery Institute













