Beta-cells Heterogeneity Uncovered by Tracing Developmental Origins
By LabMedica International staff writers
Posted on 05 Oct 2017
Heterogeneity among β-cells has recently become evident, and it is thought that this heterogeneity might play a role in the progression of diabetes. Researchers have developed a system that enables tracing of the developmental history of β-cells by genetic barcoding and multicolor imaging. In a study using the zebrafish model, they found that different histories of β-cells generate functional and proliferative heterogeneity during islet growth.Posted on 05 Oct 2017
The tracing system, called “Beta-bow”, was developed by a research team led by Dr. Nikolay Ninov, group leader at the DFG research center for Regenerative Therapies Dresden (CRTD) at Technische Universitaet Dresden (Dresden, Germany). The project was led by CRTD postdoc Sumeet Pal Singh, PhD. In addition, Sharan Janjuha (PhD-student, DIGS-BB) established the assay for calcium imaging. Additional researchers include collaborators from Daiichi Sankyo Co,Ltd (Japan), Oxford University (UK), and CRTD (Germany).
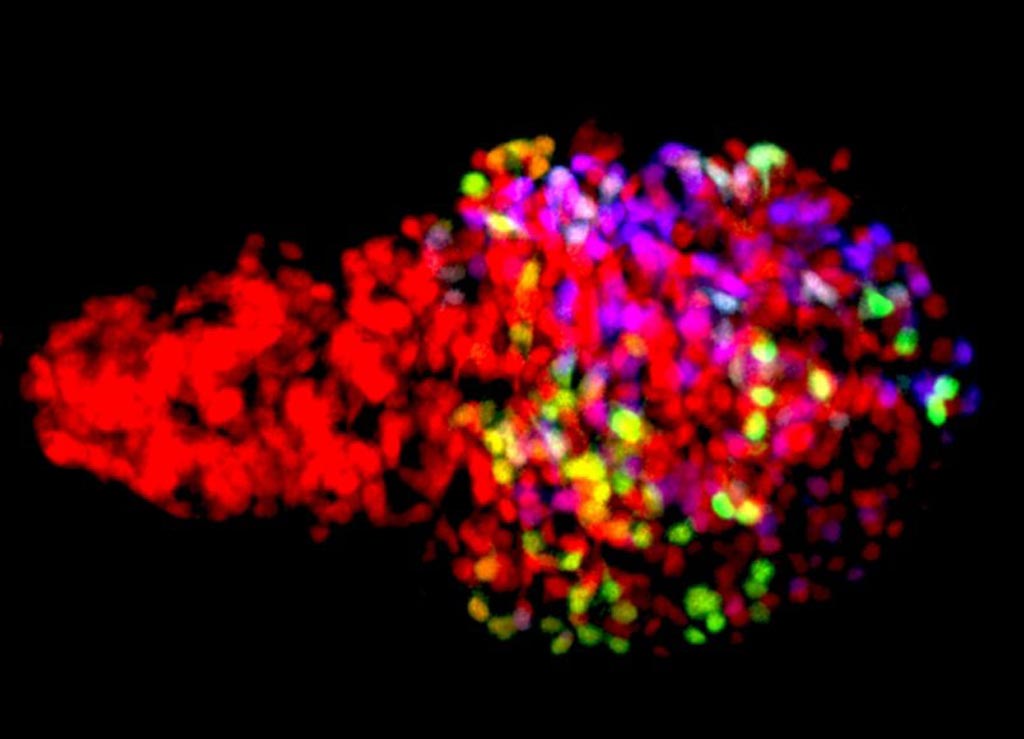
Image: Zebrafish β-cells labeled using the newly developed Beta-bow system through the combinatorial expression of fluorescent proteins, allowing the developmental history of β-cells to be traced during islet growth (Photo courtesy of and Ninov Lab, TU Dresden).
Tracing the history of individual cells in the developing organism can reveal functional differences among seemingly uniform cells. This knowledge is important for defining the characteristics of highly regenerative cells in order to target them for cellular therapies, as well as to prevent the formation of unfit cells, which compromise the overall health of the organism. The study introduced here presents a new method for tracing the history of β-cells, which perform the essential function of secreting insulin in response to glucose.
The researchers traced β-cells with regards to their proliferation, function, and time of differentiation in the zebrafish as a powerful genetic model. The study showed that β-cells with different developmental histories co-exist together, which leads to the formation of dynamic sub-populations that differ in their potential for undergoing proliferation and performing functional tasks. The study also revealed the onset of β-cell function in zebrafish, which opens new avenues to investigate how β-cells acquire a functional state using this model.
“Even 20 years after the onset of Type 1 diabetes, some β-cells can survive in the pancreas, perhaps because these cells are different from the rest, which allows them to hide from the immune system and to escape autoimmune destruction,” said Dr. Ninov. “Curiosity, and the drive to make an original contribution towards a cure for diabetes by learning more about the basic biology of β-cells,” motivates Nikolay Ninov in his daily work. The ability to directly visualize the evolution of β-cell heterogeneity in zebrafish will help to understand the dynamic regulation of β-cell sub-populations at the molecular level. This knowledge is of crucial importance for the subsequent development of effective strategies for β-cell regeneration and protection in diabetes.
“As a next step, we will use our model and cell-tracing methods to understand the signals that instruct β-cells to acquire a functional state. In particular, we found that in zebrafish this process takes only a few days after the birth of the cells, whereas it is difficult to achieve the formation of functional β-cells from human stem cells in vitro. Thus, our hypothesis is that the in vivo environment in the zebrafish pancreas provides powerful signals for rapid β-cell functional maturation. We will now identify these signals, as this knowledge can help to produce functional human β-cells in vitro for transplantation purposes,” said Dr. Ninov.
The study, by Singh SP et al, was published September 22, 2017, in the journal Nature Communications.
Related Links:
Technische Universitaet Dresden













