Monoclonal Antibody-based Vaccine Prevents Lassa Fever in Model
By LabMedica International staff writers
Posted on 22 Sep 2017
A combination of human monoclonal antibodies was used to protect monkeys infected with Lassa fever virus and prevented infection even when administered as late as eight days after exposure to the virus.Posted on 22 Sep 2017
There are no approved treatments for Lassa fever, which is endemic to the same regions of West Africa that were recently devastated by Ebola. The Lassa fever virus infects hundreds of thousands of people every year and is estimated to be fatal in approximately 34% of cases.
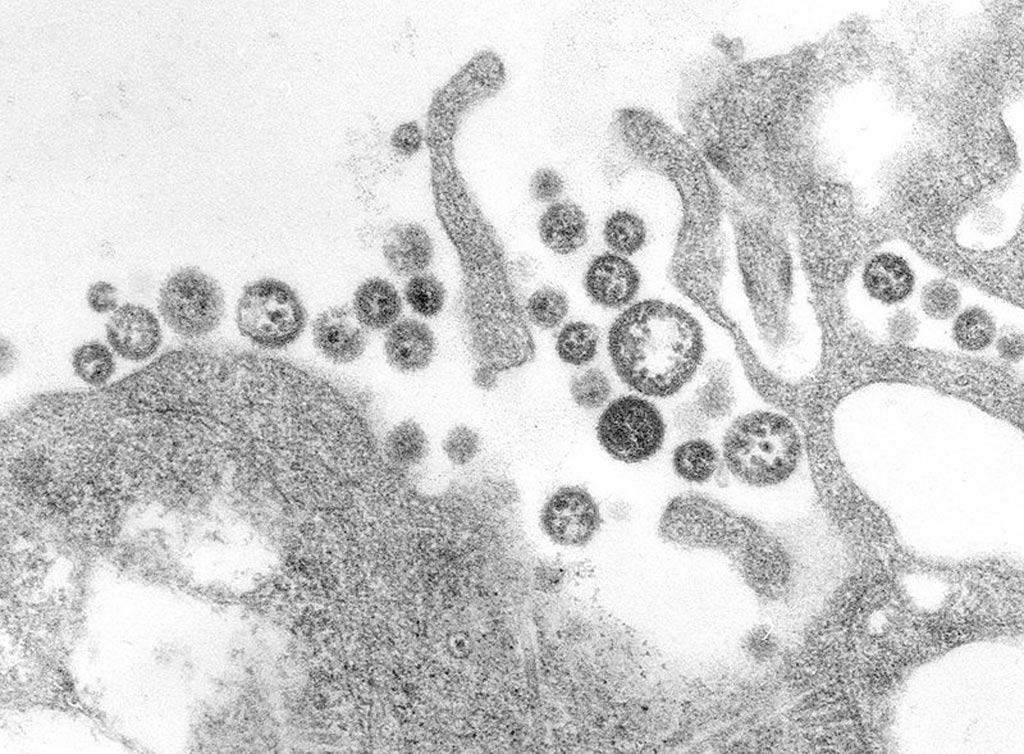
Image: A transmission electron micrograph (TEM) of a number of Lassa virus virions adjacent to some cell debris. The virus, a member of the virus family Arenaviridae, causes Lassa fever (Photo courtesy of the CDC).
Searching for a way to prevent Lassa fever, investigators at The University of Texas Medical Branch (Galveston, USA) tested a combination of human monoclonal antibodies that cross-reacted with the glycoproteins of all four clades of Lassa virus. The mixture of three monoclonal antibodies was administered to nonhuman primates (cynomolgus macaques) beginning up to eight days following exposure to a lethal dosage of Lassa virus.
Results published in the September 4, 2017, online edition of the journal Nature Medicine revealed that the treatment rescued 100% of the monkeys, even when treatment was initiated at advanced stages of disease, including up to eight days after challenge.
“In this study, we tested a combination of three monoclonal antibodies by giving them to nonhuman primates beginning up to eight days following exposure to a lethal amount of Lassa virus,” said senior author Dr. Thomas Geisbert, professor of microbiology and immunology at The University of Texas Medical Branch. “We found that the treatments were well-tolerated and provided 100% protection from Lassa fever. Without treatment, the animals show evidence of the virus in their bodies by day four after exposure.”
The investigators suggested that this treatment could benefit patients with Lassa fever in West Africa who often arrive at the clinic at a late stage of disease.
Related Links:
University of Texas Medical Branch













