Modified CRISPR Gene Editing Technique Repairs Dystrophin Mutation
By LabMedica International staff writers
Posted on 26 Apr 2017
Researchers used an alternative form of the CRISPR/Cas9 genome editing system to correct a mutation in heart cells derived from Duchenne muscular dystrophy patient skin cells and in a mouse model of the disease.Posted on 26 Apr 2017
Duchenne muscular dystrophy (DMD) is caused by mutations in the gene that encodes dystrophin, a protein crucial for maintaining muscle cell integrity and function, and the subsequent disruption of the dystrophin-associated protein complex (DAPC). The mutation occurs on the X-chromosome, and the disease effects about one of every 3,500 boys whose muscle function is so degraded that they die usually before reaching the age of 30.
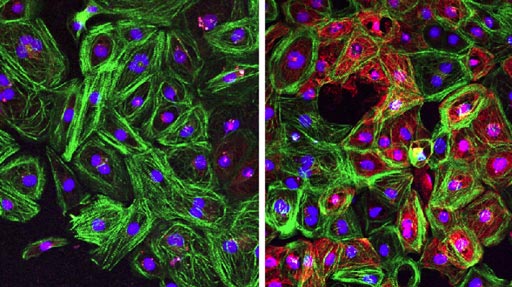
Image: Cardiomyocytes from patients with Duchenne muscular dystrophy (DMD) corrected by CRISPR-Cpf1 reframing during stemness (right) show restored dystrophin expression (red), compared to uncorrected cells (left) (Photo courtesy of Science Advances).
CRISPRs (clustered regularly interspaced short palindromic repeats) are segments of prokaryotic DNA containing short repetitions of base sequences. Each repetition is followed by short segments of "spacer DNA" from previous exposures to a bacterial virus or plasmid. CRISPRs are found in approximately 40% of sequenced bacteria genomes and 90% of sequenced archaea. CRISPRs are often associated with cas genes that code for proteins related to CRISPRs. Since 2013, the CRISPR/Cas system has been used in research for gene editing (adding, disrupting, or changing the sequence of specific genes) and gene regulation. By delivering the Cas9 enzyme and appropriate guide RNAs into a cell, the organism's genome can be cut at any desired location. The conventional CRISPR/Cas9 system is composed of two parts: the Cas9 enzyme, which cleaves the DNA molecule and specific RNA guides (CRISPRs) that shepherd the Cas9 protein to the target gene on a DNA strand.
Investigators at the University of Texas Southwestern Medical Center used a modified version of CRISPR gene editing called CRISPR/Cpf1 to edit the genome of cardiomyocytes derived from DMD patient skin cells and in mdx mice, an animal model of DMD. CRISPR/Cpf1 differs from CRISPR/Cas9 in a number of key ways. Cpf1 is much smaller than the Cas9 enzyme, which makes it easier to package inside a virus and therefore easier to deliver to muscle cells. It also recognizes a different sequence of DNA than Cas9 does, which provides greater flexibility in terms of use.
The investigators reported in the April 12, 2017, online edition of the journal Science Advances that CRISPR/Cpf1 genomic editing of human iPSCs (induced pluripotent stem cells) - either by skipping an out-of-frame DMD exon or by correcting a nonsense mutation - restored dystrophin expression after differentiation to cardiomyocytes and enhanced contractile function. Similarly, pathophysiological hallmarks of muscular dystrophy were corrected in mdx mice following Cpf1-mediated germline editing.
"We took patient-derived cells that had the most common mutation responsible for Duchenne muscular dystrophy and we corrected them in vitro to restore production of the missing dystrophin protein in the cells. This work provides us with a promising new tool in the CRISPR toolbox," said senior author Dr. Eric Olson, professor of molecular biology at the University of Texas Southwestern Medical Center. "There will be some genes that may be difficult to edit with Cas9 but may be easier to modify with Cpf1, or vice versa. The two proteins have different biochemical properties and recognize different DNA sequences, so these properties create more options for gene editing. CRISPR/Cpf1 gene editing can be applied to a vast number of mutations in the dystrophin gene. Our goal is to permanently correct the underlying genetic causes of this terrible disease, and this research brings us closer to realizing that end."













