Heating Method Developed for Rapid Delivery of Macromolecules
By LabMedica International staff writers
Posted on 04 Apr 2017
A team of cancer researchers developed a precise and controlled mechanism that rapidly introduces molecules as large as proteins or nucleic acids into living cells.Posted on 04 Apr 2017
Previously, investigators at Harvard University had shown that gold, pyramid-shaped microstructures could focus nanosecond laser pulses into electromagnetic hotspots.
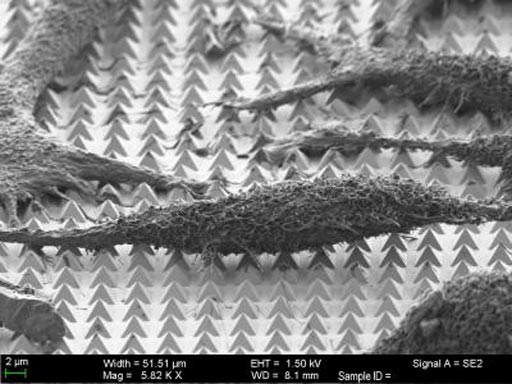
Image: A scanning-electron microscope (SEM) image of chemically-fixed HeLa cancer cells on the gold nanopyramid substrate. Following nanosecond laser pulses, the tips of the pyramids create tiny holes in the cell membranes, allowing molecular cargo to diffuse into the cells (Photo courtesy of Harvard University).
The investigators cultured HeLa cells directly on top of these pyramid structures in a growth solution containing specific cargo molecules. They focused a laser on the pyramids, and nanopulses of the laser light caused the hotspots at the tips of the pyramids to warm to a temperature of about 300 degrees Celsius. This localized heating generated bubbles at the tip of each pyramid. The bubbles inserted themselves into the membranes of the HeLa cells, opening brief pores, which allowed the target molecules to diffuse into the cells. The pores sealed very quickly, and the cells healed themselves and remained alive and dividing for an extended period. Clinically, this method could be used in ex vivo therapies, where unhealthy cells are taken out of the body, loaded with a drug or DNA, and reintroduced into the body.
The investigators optimized the fabrication technique to produce plasmonic structures that were ultrasmooth and precisely patterned over large areas. They used flow cytometry to characterize the delivery efficiency of cargos ranging in size from 0.6 to 2000 kiloDaltons into cells (up to 95% for the smallest molecule) and viability of cells (up to 98%).
The technique offered a throughput of 50,000 cells per minute, which could be scaled up as necessary. This technique was cost-effective, as each large-area photolithography substrate could be used to deliver cargo molecules to millions of cells, and switching to a nanosecond laser rendered the setup cheaper and easier to use. Furthermore, this approach offered additional desirable features such as spatial selectivity, reproducibility, minimal residual fragments, and cost-effective fabrication.
"It is great to see how the tools of physics can greatly advance other fields, especially when it may enable new therapies for previously difficult to treat diseases," said senior author Dr. Eric Mazur, professor of physics and applied physics at Harvard University.
The gold nanopyramid technique for molecular loading of living cells was described in the March 14, 2017, online edition of the journal ACS Nano.













