HTS Method Based on 3D Tumor Organoid Cultures
By LabMedica International staff writers
Posted on 30 Apr 2018
A drug development team has described a high-throughput screening (HTS)-compatible method – based on three-dimensional (3D) tumor organoids – for evaluating multiple chemical compounds for potential chemotherapeutic drug candidates.Posted on 30 Apr 2018
Traditional high-throughput drug screening in cancer research routinely relies on two-dimensional cell models, which inadequately recapitulate the physiologic context of cancer. Three-dimensional cell models are thought to better mimic the complexity of in vivo tumors. Numerous methods to culture three-dimensional organoids have been described, but most are nonhomogeneous and expensive, and hence impractical for high-throughput screening (HTS) purposes.
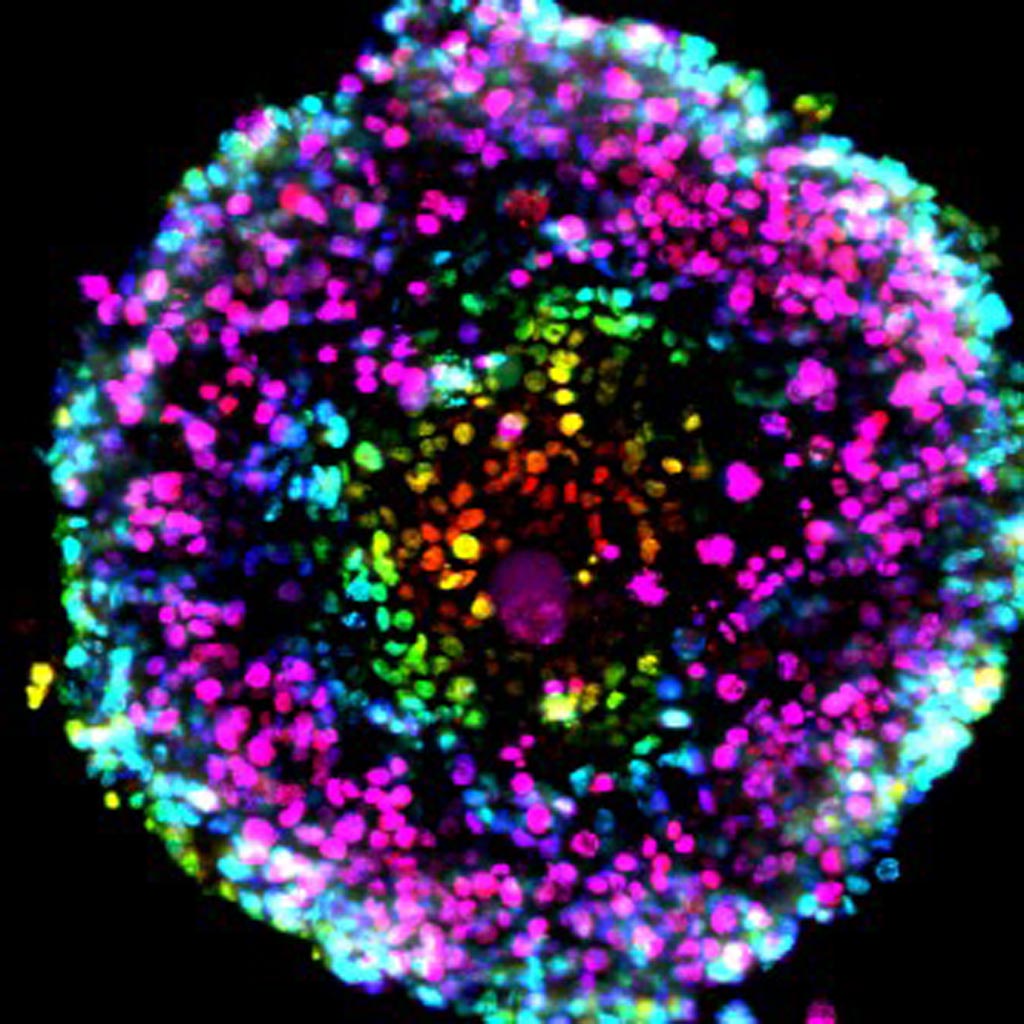
Image: A micrograph of a pancreatic cancer spheroid culture (Photo courtesy of Dr. Shurong Hou, Scripps Research Institute).
Investigators at the Scripps Research Institute (Jupiter, FL, USA) sought to develop an improved screening method based on three-dimensional organoids. To this end, the described in the April 19, 2018, online edition of the journal SLAS Discovery an HTS-compatible method that enabled the consistent production of organoids in standard flat-bottom 384- and 1536-well plates by combining the use of a cell-repellent surface with a bio-printing technology incorporating magnetic force.
This novel method combined specialized high-density microtiter plates formulated with an ultra-low attachment surface along with gold nanoparticles (nanoshuttles), which were used to label cancer cells in vitro. Once labeled, a magnet assembled the cells into a three-dimensional spheroid or organoid structure. This three-dimensional structure was retained, and chemical compounds were added to assess their therapeutic efficacy.
The investigators validated this process by evaluating the effects of well-characterized anticancer agents against four patient-derived pancreatic cancer KRAS mutant-associated primary cells, including cancer-associated fibroblasts. The technology was tested for its compatibility with HTS automation by completing a cytotoxicity pilot screen of around 3300 approved drugs.
Data obtained during the study indicated that the technique could be readily applied to support large-scale drug screening relying on clinically relevant, three-dimensional tumor models directly harvested from patients, an important milestone toward personalized medicine.
Related Links:
Scripps Research Institute













