Inhibiting Fibronectin Polymerization Reduces Injury to Cardiac Muscle
By LabMedica International staff writers
Posted on 24 Apr 2018
Working with a mouse model of human heart disease, researchers showed that interfering with fibronectin (FN) polymerization or its genetic deletion in fibroblasts would attenuate cardiac myofibroblasts (MF), fibrosis, and improve cardiac function following heart attack related injury.Posted on 24 Apr 2018
Fibronectin is a high-molecular weight glycoprotein of the extracellular matrix that binds to integrin receptor proteins. Similar to integrins, fibronectin binds extracellular matrix components such as collagen, fibrin, and heparan sulfate proteoglycans. Fibronectin exists as a protein dimer, consisting of two nearly identical monomers linked by a pair of disulfide bonds. The fibronectin protein is produced from a single gene, but alternative splicing of its pre-mRNA leads to the creation of several isoforms. Fibronectin plays a major role in cell adhesion, growth, migration, and differentiation, and it is important for processes such as wound healing and embryonic development. Altered fibronectin expression, degradation, and organization have been associated with a number of pathologies, including cancer and fibrosis.
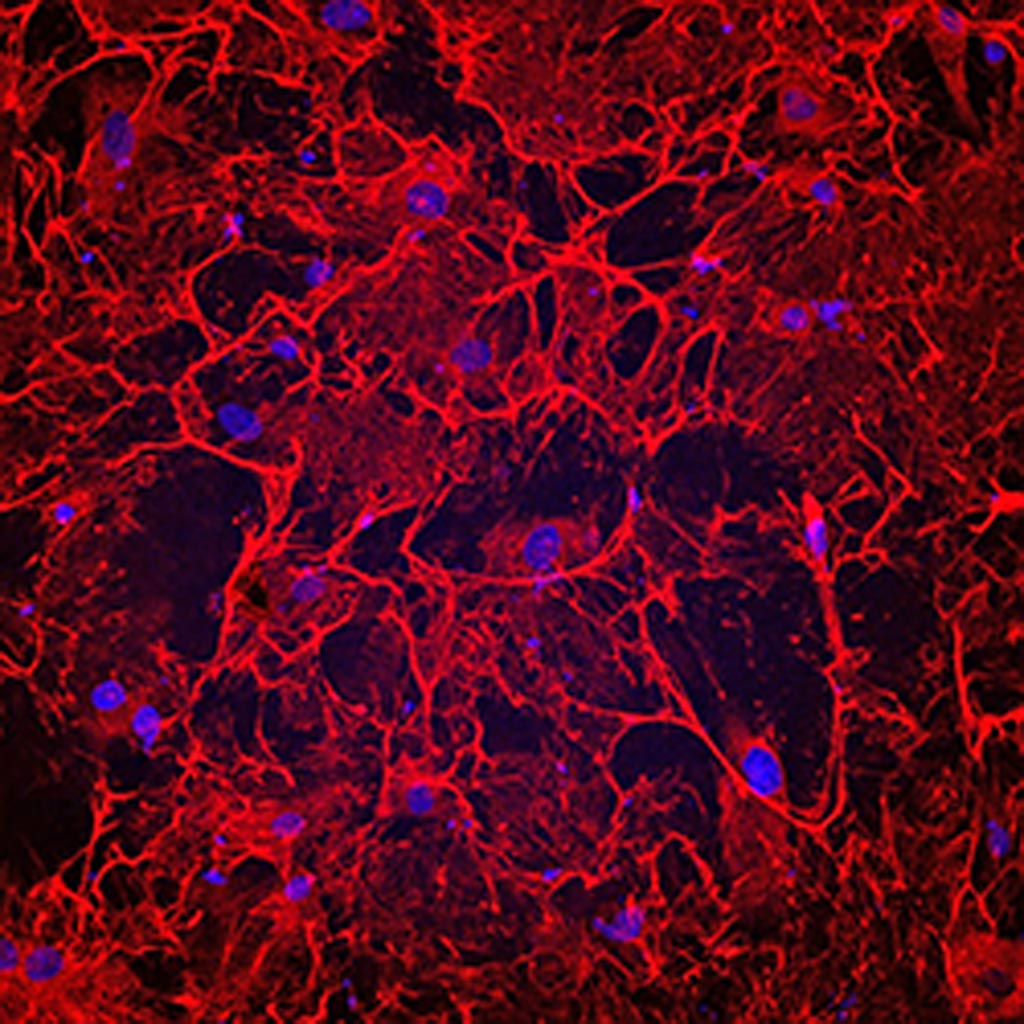
Image: A photomicrograph showing fibrotic heart cells from a patient who had heart failure. The cells have an elaborate fibronectin matrix (shown in red), which causes fibrosis and heart damage (Photo courtesy of Cincinnati Children\'s Hospital Medical Center).
Fibronectin (FN) polymerization is necessary for collagen matrix deposition and is a key contributor to increased abundance of cardiac myofibroblasts (MF) following cardiac injury. To better understand the role of FN polymerization, investigators at Cincinnati Children's Hospital Medical Center (OH, USA) used the synthetic polymerization inhibitor peptide pUR4 to assess the impact of blocking FN polymerization on pathologic cellular features such as proliferation, migration, extracellular matrix (ECM) deposition, and associated mechanisms.
To evaluate the therapeutic potential of inhibiting FN polymerization in vivo, wildtype (WT) mice received daily intraperitoneal injections of either pUR4 or a control peptide (III-11C) immediately after cardiac surgery, for seven consecutive days. Mice were analyzed seven days post-injury to assess myofibroblast markers and inflammatory cell infiltration, or four weeks post-injury, to evaluate long-term effects of FN inhibition on cardiac function and fibrosis. Further, inducible, fibroblast-restricted, FN gene ablated mice were utilized to evaluate cell specificity of FN expression and polymerization in the heart.
Results published in the April 13, 2018, online edition of the journal Circulation revealed that pUR4 administration on activated MF reduced FN and collagen deposition into the ECM and attenuated cell proliferation, likely mediated through decreased c-myc signaling. The pUR4 peptide also enhanced fibroblast migration accompanied by increased beta-1 integrin internalization and reduced levels of phosphorylated focal adhesion kinase (FAK) protein. Daily administration of pUR4 in vivo for seven days following injury significantly reduced MF markers and neutrophil infiltration. This treatment regimen also significantly lessened myocardial dysfunction, pathologic cardiac remodeling, and fibrosis up to four weeks post-injury. Finally, inducible ablation of FN in fibroblasts post-injury resulted in significant functional cardio-protection with reduced hypertrophy and fibrosis.
"Our data are a strong proof of principle and the first to show that inhibiting fibronectin polymerization preserves heart function, reduces left ventricle remodeling and limits formation of fibrotic connective tissue," said senior author Dr. Burns Blaxall, director of translational research in the heart institute at Cincinnati Children's Hospital Medical Center.
Related Links:
Cincinnati Children's Hospital Medical Center













